Anyone who’s ever seen a NICU or statistics on the health outcomes for extremely premature babies understands how artificial womb technology could be incredibly impactful.
At the same time, it poses some tricky ethical challenges:
- How can you get informed, ethical consent to trial this? It’s nigh impossible to predict who will need a premature delivery.
- In places like the US with limitations / controversy on reproductive rights, how do you push this forward without impacting assessments of “fetal viability” that may impact the abortion debate?
Great piece in Nature News below 👇🏻
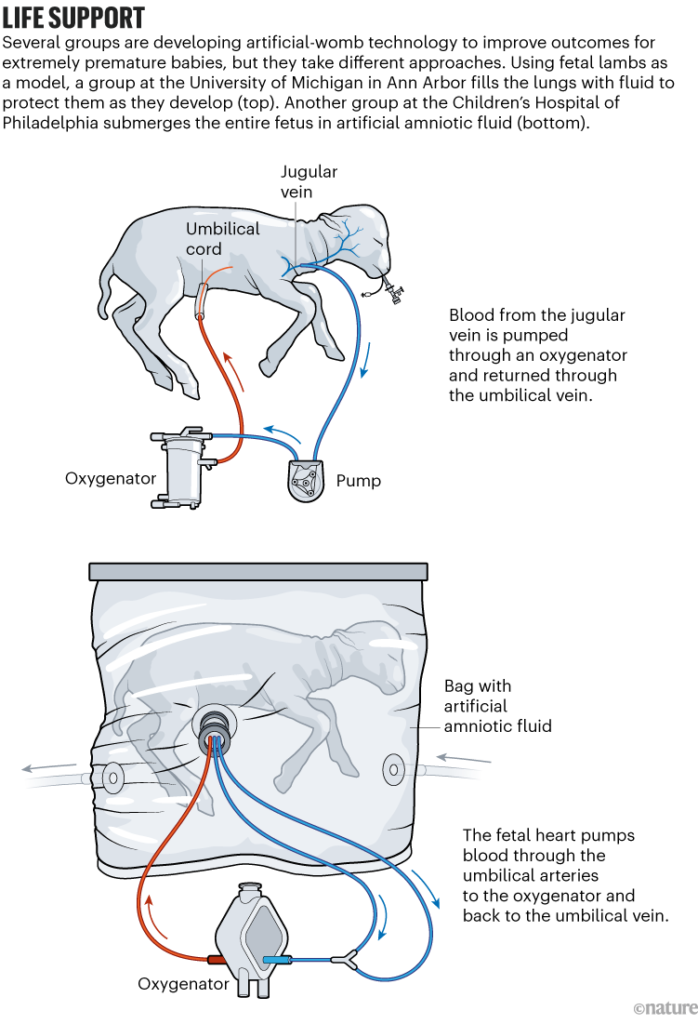
Now, the researchers at CHOP are seeking approval for the first human clinical trials of the device they’ve been testing, named the Extra-uterine Environment for Newborn Development, or EXTEND. The team has emphasized that the technology is not intended — or able — to support development from conception to birth. Instead, the scientists hope that simulating some elements of a natural womb will increase survival and improve outcomes for extremely premature babies. In humans, that’s anything earlier than 28 weeks of gestation — less than 70% of the way to full term, which is typically between 37 and 40 weeks.
The CHOP group has made bold predictions about the technology’s potential. In another 2017 video describing the project, Alan Flake, a fetal surgeon at CHOP who has been leading the effort, said: “If it’s as successful as we think it can be, ultimately, the majority of pregnancies that are predicted at-risk for extreme prematurity would be delivered early onto our system rather than being delivered premature onto a ventilator.” In 2019, several members of the CHOP team joined a start-up company, Vitara Biomedical in Philadelphia, which has since raised US$100 million to develop EXTEND. (Flake declined to comment for this article, citing “conflicts of interest” and “restrictions on proprietary information”. His co-authors on the 2017 paper did not respond to Nature’s request for comment.)

Leave a Reply