Huntington’s Disease is one of the classic “uncurable” neurodegenerative diseases. A genetic mutation produces a mutant form of a protein (called Huntingtin) which leads to sudden onset of symptoms which gets progressively worse until death. It’s so bad that it’s considered a “textbook” example of the need for genetic counseling when people are considering getting their DNA sequenced: for some, it’s better to not know they’re a carrier or have the disease.
But some exciting news from biotech company uniQure today may change that! UniQure announced the results of a Phase I/II study showing how a revolutionary gene therapy + brain surgery can slow disease progression by 75% over 36 months.
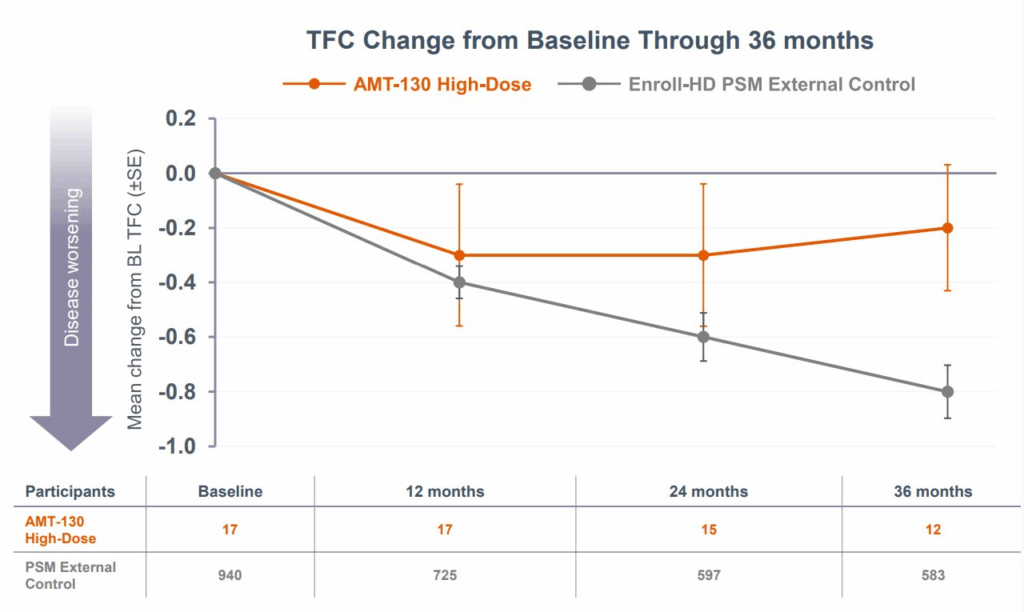
What also stood out to me was the use of a synthetic control arm — basically using data collected on real world patients (rather than trial participants) to alleviate the time and cost burden of needing to recruit from a relatively rare disease population (as well as capture disease progression in a realistic setting). Overall, an amazing feat for everyone involved and hopefully a beacon of hope for those with Huntington’s Disease or carrying the gene.

Pivotal Phase I/II AMT-130 Huntington’s Disease Update [PDF]
uniQure presentation
