Unless you just discovered YouTube yesterday, you’ve probably seen countless videos of (and maybe even have tried?) the infamous Diet Coke + Mentos reaction… which brings us to the subject of this month’s paper.
An enterprising physics professor from Appalachian State University decided to have her sophomore physics class take a fairly rigorous look at what drives the Diet Coke + Mentos reaction and what factors might influence its strength and speed. They were not only able to publish their results in the American Journal of Physics, but the students were also given an opportunity to present their findings in a poster session (Professor Coffey reflected on the experience in a presentation she gave). In my humble opinion, this is science education at its finest: instead of having students re-hash boring experiments which they already know the results of, this allowed them to do fairly original research in a field which they probably had more interest in than in the typical science lab course.
So, what did they find?
The first thing they found is that it’s not an acid-base reaction. A lot of people, myself included, believe the diet coke + Mentos reaction is the same as the baking soda + vinegar “volcano” reactions that we all did as kids. Apparently, we were dead wrong, as the paper points out:
The pH of the diet Coke prior to the reaction was 3.0, and the pH of the diet Coke after the mint Mentos reaction was also 3.0. The lack of change in the pH supports the conclusion that the Mint Mentos–Diet Coke reaction is not an acid-base reaction. This conclusion is also supported by the ingredients in the Mentos, none of which are basic: sugar, glucose, syrup, hydrogenated coconut oil, gelatin, dextrin, natural flavor, corn starch, and gum arabic … An impressive acid-base reaction can be generated by adding baking soda to Diet Coke. The pH of the Diet Coke after the baking soda reaction was 6.1, indicating that much of the acid present in the Diet Coke was neutralized by the reaction.
Secondly, the “reaction” is not chemical (no new compounds are created), but a physical response because the Mentos makes bubbles easier to form. The Mentos triggers bubble formation because the surface of the Mentos is itself extremely rough which allows bubbles to aggregate (like how adding string/popsicle stick to an oversaturated mixture of sugar and water is used to make rock candy). But that doesn’t explain why the Mentos + Diet Coke reaction works so well. The logic blew my mind but, in retrospect, is pretty simple. Certain liquids are more “bubbly” by nature – think soapy water vs. regular water. Why? Because the energy that’s needed to form a bubble is lower than the energy available from the environment (e.g., thermal energy). So, the question is, what makes a liquid more “bubbly”? One way is to heat the liquid (heating up Coke makes it more bubbly because heating the carbon dioxide inside the soda gives the gas more thermal energy to draw upon), which the students were able to confirm when they looked at how much mass was lost during a Mentos + Diet coke reaction under three different temperatures (Table 3 below):
What else? It turns out that what other chemicals a liquid has dissolved is capable of changing the ease at which bubbles are made. Physicists/chemists will recognize this “ease” as surface tension (how tightly the surface of a liquid pulls on itself) which you can see visually as a change in the contact angle (the angle that the bubble forms against a flat surface, see below):
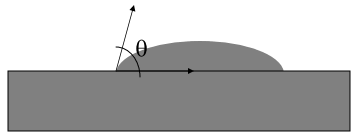
The larger the angle, the stronger the surface tension (the more tightly the liquid tries to pull in on itself to become a sphere). So, what happens when we add the artificial sweetener aspartame and potassium benzoate (both ingredients in Diet Coke) to water? As you can see in Figure 4 below, the contact angle in (b) [aspartame] and (c) [potassium benzoate] are smaller than (a) [pure water]. Translation: if you add aspartame and/or potassium benzoate to water, you reduce the amount of work that needs to be done by the solution to create a bubble. Table 4 below that shows the contact angles of a variety of solutions that the students tested as well as the amount of work needed to create a bubble relative to pure water:
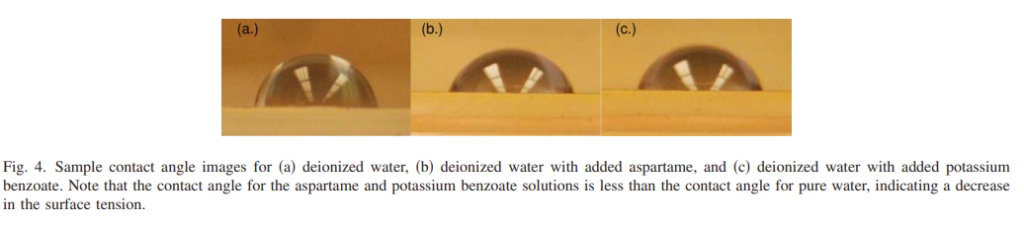
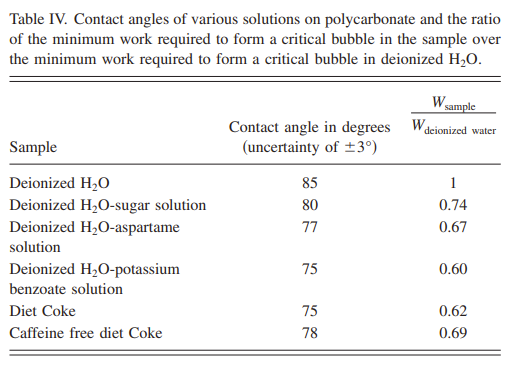
This table also shows why you use Diet Coke rather than regular Coke (basically sugar-water) to do the Mentos thing – regular coke has a higher contact angle (and ~20% more energy needed to make a bubble).
Another factor which the paper considers is how long it takes the dropped Mentos to sink to the bottom. The faster a Mentos falls to the bottom, the longer the “average distance” that a bubble needs to travel to get to the surface. As bubbles themselves attract more bubbles, this means that the Mentos which fall to the bottom the fastest will have the strongest explosions. As the paper points out:
The speed with which the sample falls through the liquid is also a major factor. We used a video camera to measure the time it took for Mentos, rock salt, Wint-o-Green Lifesavers, and playground sand to fall through water from the top of the water line to the bottom of a clear 2 l bottle. The average times were 0.7 s for the Mentos, 1.0 s for the rock salt and the Lifesavers, and 1.5 s for the sand … If the growth of carbon dioxide bubbles on the sample takes place at the bottom of the bottle, then the bubbles formed will detach from the sample and rise up the bottle. The bubbles then act as growth sites, where the carbon dioxide still dissolved in the solution moves into the rising bubbles, causing even more liberation of carbon dioxide from the bottle. If the bubbles must travel farther through the liquid, the reaction will be more explosive.
So, in conclusion, what makes a Diet Coke + Mentos reaction stronger?
- Temperature (hotter = stronger)
- Adding substances which reduce the surface tension/contact angle
- Increasing the speed at which the Mentos sink to the bottom (faster = stronger)
I wish I had done something like this when I was in college! The paper itself also goes into a lot of other things, like the use of an atomic force microscope and scanning electron microscopes to measure the “roughness” of the surface of the Mentos, so if you’re interested in additional things which can affect the strength of the reaction (or if you’re a science teacher interested in coming up with a cool project for your students), I’d strongly encourage taking a look at the paper!
Paper: Coffey, T. “Diet Coke and Mentos: What is really behind this physical reaction?”. American Journal of Physics 76:6 (Jun 2008) – doi: 10.1119/1.2888546
